Software for Remote Clinical Trial Monitoring
Architecture, Technologies, Outcomes
In healthcare IT since 2005, ScienceSoft helps research organizations and pharmaceutical and biotech companies build impactful remote trial monitoring solutions that provide full visibility into site compliance and performance while reducing monitoring costs.
A Shift to Remote and Centralized Monitoring in Clinical Trials
Biotech and pharmaceutical companies, CROs, and research organizations are increasingly shifting away from the traditional on-site monitoring model. It is being replaced by remote clinical monitoring (without CRA visits to research sites) and centralized monitoring (providing CRA with centralized access to aggregated monitoring data).
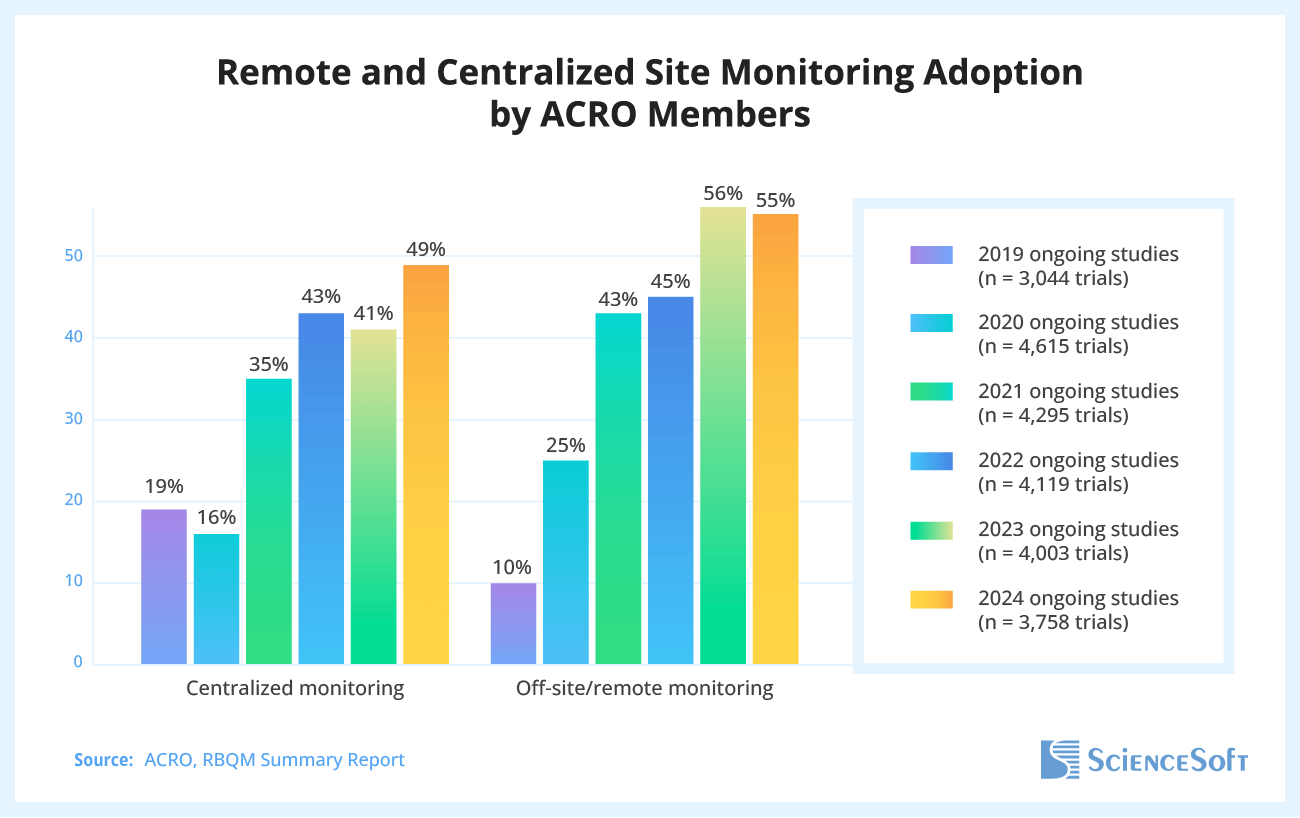
A five-year survey by the Association of Clinical Research Organizations (ACRO), which analyzed nearly 4,000 clinical trials, found that from 2019 to 2023, the use of remote and centralized clinical site monitoring grew by 460% and 116%, respectively.
Software for Remote Clinical Trial Monitoring: Essentials
Software for remote clinical trial monitoring allows clinical research associates (CRAs) to perform all site oversight activities, including visits, remotely. Such solutions focus on consolidating disparate information across sites and systems, gathering and integrating clinical data along with operational and compliance indicators, to give CRAs full visibility into investigator performance.
Such solutions can be powered by big data tech and AI/ML analytics to detect anomalies and risk factors by examining historical and real-time trial data. This functionality helps CRAs focus on high-risk sites, documents, and processes, which helps research organizations reduce trial costs, timelines, and manual routines.
Sample Architecture for Remote Trial Monitoring Software
Below, ScienceSoft's software engineering experts describe the key architecture elements and data flows of a sample solution for centralized and remote monitoring in clinical trials that meets the essential needs of most research organizations.
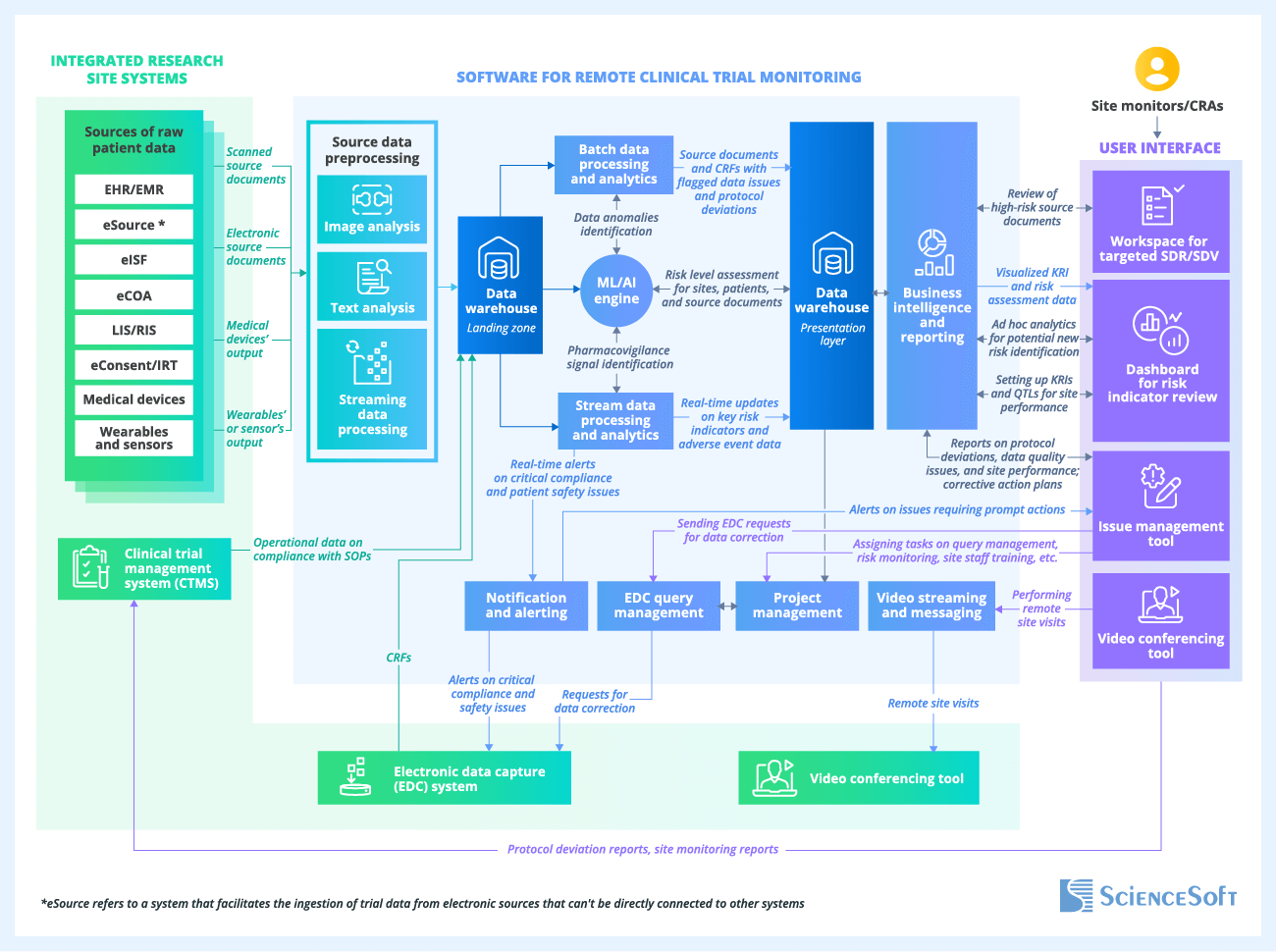
Source data ingestion and preprocessing
The software receives electronic case report forms (eCRFs), operational data on the sites’ compliance with protocol SOPs, and source documents, such as informed consent forms (ICFs), lab test results, and X-rays, from integrated systems. The solution can ingest source patient health data from text, image, audio, and video files, along with the readings from wearable devices used for remote patient monitoring in clinical trials. Clinical data may also include standardized coding, such as ICD-10 for diagnoses, CPT for procedures, as well as structured CCDA summaries. Additionally, sites can upload scanned copies of paper source documents, such as reports from external laboratories.
Once ingested, the data is sent to a preprocessing module that automatically removes patient-identifying details, performs optical character recognition (OCR) for the content of scanned documents (including handwritten notes and signatures), and transcribes audio recordings, among other tasks. After that, the data goes into the landing zone of the data warehouse, which serves as a repository for raw trial data.
Automated source document verification and review (SDV/SDR)
The batch data processing and analytics module extracts CRFs and preprocessed source documents from the raw clinical data repository (the landing zone) and performs automatic SDV/SDR. This includes verifying CRF data against the source documents (identifying transcription errors), checking CRF data quality (detecting blank fields, missing signatures and dates, inconsistencies, etc.), and confirming compliance with protocol and SOP requirements.
Real-time source clinical data analysis
In addition to the batch processing and analysis module used for scheduled analysis, incoming CRFs and source documents are processed by the stream data processing module in real time. This enables CRAs to constantly monitor key risk indicators (KRIs) associated with site performance, trial performance, and patient safety. If potential high-risk threats are identified (e.g., previously unknown adverse effects), the module alerts sites and CRAs through a notification and alerting service.
After the batch and real-time source data processing, highly structured clinical data, which is ready for complex analytics, is stored in the presentation layer of the data warehouse.
ML/AI-driven analytics
The machine learning (ML) and artificial intelligence (AI) engine analyzes trends in CRF data to identify anomalies and outliers that may indicate issues with compliance and data quality at a particular site. For instance, lower adverse event rates at a specific site compared to others may suggest potential underreporting.
The ML/AI engine also processes incoming site data in real time to identify pharmacovigilance signals and compliance issues that require urgent attention.
Lastly, the engine uses data about identified errors, clinical data defects, and protocol deviations to assign a risk level to specific sites or document types. For example, it can assign a high-risk status to sites with frequent data gaps or with recurring discrepancies in investigational drug accountability records.
Data visualization and reporting
From the structured clinical data repository (the presentation layer of the data warehouse), the analyzed source data is directed to the business intelligence (BI) and reporting module, which visualizes and presents it to CRAs in the form of interactive dashboards. These dashboards offer convenient tools for data exploration (e.g., filtering, searching, and drill-down capabilities), report generation, and routine remote data monitoring.
All the CRFs and source documents with errors, defects, and anomalies that were flagged after automatic checks are available in a dedicated workspace for SDV/SDR. Here, CRAs can manually review the source documents that are critically important (e.g., serious adverse event reports) or have a high-risk status.
The BI module also sends real-time KRI updates and the results of ML/AI-driven risk analysis to the risk indicator review dashboard. Here, CRAs can dig into the source data associated with each potential risk, establish new KRIs, and adjust QTLs based on their findings.
Monitoring results sharing and corrective actions
Reports on the results of remote monitoring and audits with all the documented protocol deviations are transferred to the integrated clinical trial management system (CTMS), where they become available to site staff.
A project management module is connected with an issue management tool in the user interface, enabling CRAs to create tasks based on their action plans for mitigating risks and protocol deviations. These tasks become available to their team members, project managers, and data managers for assignment, execution, and monitoring.
Via an EDC query management module, CRAs can send queries to investigators to correct errors discovered during monitoring and then track the resolution of these queries. A video conferencing tool lets CRAs conduct remote monitoring visits to oversee SOP compliance and execute corrective actions, such as site staff training.
Key Techs and Tools We Use for Remote Trial Monitoring Solutions
Data security and governance
Reported Outcomes of Implementing Software for Remote Clinical Trial Monitoring
LabCorp, a leading US diagnostic and drug discovery company, measured the impact of centralized monitoring technology on the site oversight efficiency, timing, and costs across 12 studies conducted by its Drug Development division over a four-year period.
* Compared to all other studies conducted during the same period using traditional on-site monitoring.
LabCorp’s researchers highlighted the following benefits of centralized monitoring software:
Fewer site visits and other monitoring activities
The software's analytical capabilities enabled the automatic evaluation of site performance based on multiple operational and clinical metrics, assigning each site a risk score ranging from 1 to 4. LabCorp’s Drug Development division aligned the monitoring intervention level (such as the frequency of visits and quality audits) with this score, preventing excessive activities at successful sites.
Decreased overall risk level in the trial
The technology enabled monitors to focus all their attention on underperforming sites and timely implement actions to control and train staff there. As a result, despite a decrease in total monitoring efforts, site compliance did not decline but, instead, kept improving, with some sites showing a dramatic reduction in risk scores.
Improved timelines for monitoring processes
An environment for collaborative work on aggregated, statistically processed, and visualized information made the joint data review much easier for experts, including medical, statistical, operational, and data quality reviewers. Furthermore, it streamlined the collaboration among central monitors, site monitors, and project managers, which helped to accelerate data issue resolution.
How Much Does It Cost to Develop Custom Software for Remote Clinical Trial Monitoring?
The cost of implementing custom software for remote clinical trial monitoring ranges from $150,000 to over $600,000, depending on the project scope.
Costs mainly depend on the number of data sources, integration difficulty, and functional complexity (including AI-supported functionality). Below are ballpark estimates for building a custom solution for remote clinical trial monitoring with basic, standard, and advanced capabilities.
Basic
From $150,000
Includes core capabilities such as:
- Up to 5 integrated data sources (e.g., EDC, CTMS, eConsent, eCOA, LIS/RIS).
- Rule-based, batch processing for medical coding, source data validation, and risk assessment.
- Targeted SDV/SDR and basic risk analytics.
- EDC query management.
Standard
From $ 300,000
In addition to all basic capabilities, this tier offers:
- Integrations with EHR/EMR.
- Support for streaming data processing and real-time analytics.
- Integration of an external video conferencing service.
- AI-driven detection of data quality problems.
Advanced
From $ 600,000
In addition to all standard capabilities, this tier offers:
- Integrations with medical sensors and wearable devices.
- Custom video conferencing tool enabling automatic AI-based documentation of remote monitoring visits.
- AI-based predictive modeling of site performance for advanced risk analytics.
Need a more precise figure?
Answer a few questions to get a free and non-binding quote for your project from ScienceSoft's experts.
Why Entrust Your Remote Clinical Trial Monitoring System to ScienceSoft
- Since 2005 in healthcare software development.
- 150+ successful projects in the domain.
- Since 1989 in data analytics, data science, and AI.
- Experience in meeting GCP, FDA, HIPAA, HITECH, GDPR, and 21st Century Cures Act requirements.
- Proven expertise in FHIR and USCDI-based interoperability, as well as medical data standards (SNOMED CT, LOINC, RxNorm).
- An in-house Architecture Center of Excellence to ensure the optimal balance of performance, cost, and scalability for clinical trial solutions.
Our awards and partnerships
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row
Recognized on Newsweek’s 2025 America’s Most Reliable Companies List
Recognized by Health Tech Newspaper awards for the third time

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Named Best in Class in Medical Device Connectivity by Frost & Sullivan (2023)
Listed in IAOP’s 2025 Global Outsourcing 100 for the 4th year running
ISO 13485-certified quality management system
ISO 27001-certified security management system











