Healthcare Software Product Development
A Guide for Product Companies
Since 2005, ScienceSoft has helped medical software providers and healthtech startups design and develop scalable, user-friendly solutions for the healthcare industry. We engineer applications of varying complexity, from AI-powered chatbots to add a competitive advantage to your app to full-fledged SaMD for particular medical specializations.
Healthcare Software Product Development: Summary
Healthcare software product development is the full cycle of creating digital health solutions, from early design to certification, launch, and ongoing support. The scope ranges from patient apps and clinician tools to AI triage systems and software as a medical device (SaMD).
Going beyond feature delivery, the process requires navigating a regulated environment where safety, privacy, and clinical logic guide every technical decision. To deliver successful, market-ready healthcare applications, product companies and startups should deeply understand healthcare regulations, such as HIPAA, GDPR, and the 21st Century Cures Act, as well as medical software requirements set by regulatory bodies like the FDA (US) and MDR (EU). Proficiency in healthcare data exchange standards, such as HL7 and FHIR, is equally important, along with strong domain knowledge to address the diverse expectations of clinicians, patients, and administrative staff.
Key steps to develop healthcare software:
For clinical or regulated use: clinical and market discovery, regulatory classification and compliance planning, architecture design focused on PHI security and interoperability, UX and UI design optimized for clinical workflows and patient safety, Agile MVP development, security testing, regulatory submission, production rollout, and clinical onboarding, post-launch optimization.
For non-clinical use: market and user research, privacy and regulatory scoping, secure, scalable architecture design, UX and UI design focused on user engagement and accessibility, Agile MVP development, QA and compliance checks, launch and user onboarding, continuous updates, and analytics.
- Timelines: 3–12+ months.
- Cost: $105,000–$300,000 for an MVP of a full-fledged app, depending on the complexity. Use our free online calculator to get a custom quote from our consultants.
- Team: a project manager, a compliance consultant, a business analyst, a system architect, a DevOps engineer, UX and UI designers, front-end and back-end developers, and QA engineers.
ScienceSoft's team helps healthcare startups transform their ideas into safe, compliant, and ready-to-use software.
We start by identifying real problems in the healthcare settings that you want to solve. Next, we analyze your competitors and assess whether your idea is technically feasible. We also check what healthcare regulations apply to your future product. Then, we develop a business case that includes compliance costs and projected ROI. Based on this, we create a development plan that aligns with the requirements of HIPAA, GDPR, FDA 510(k), or MDR.
Whether you want to build a new AI-powered clinical decision support tool or enhance your existing app with intelligent features like an AI voice scheduling agent, pre-procedure preparation assistant, or a fall risk prediction module, we can help you navigate regulatory pathways smoothly, launch quickly, and drive consistent adoption among clinicians, administrative staff, or patients.
Healthcare Software Market Overview
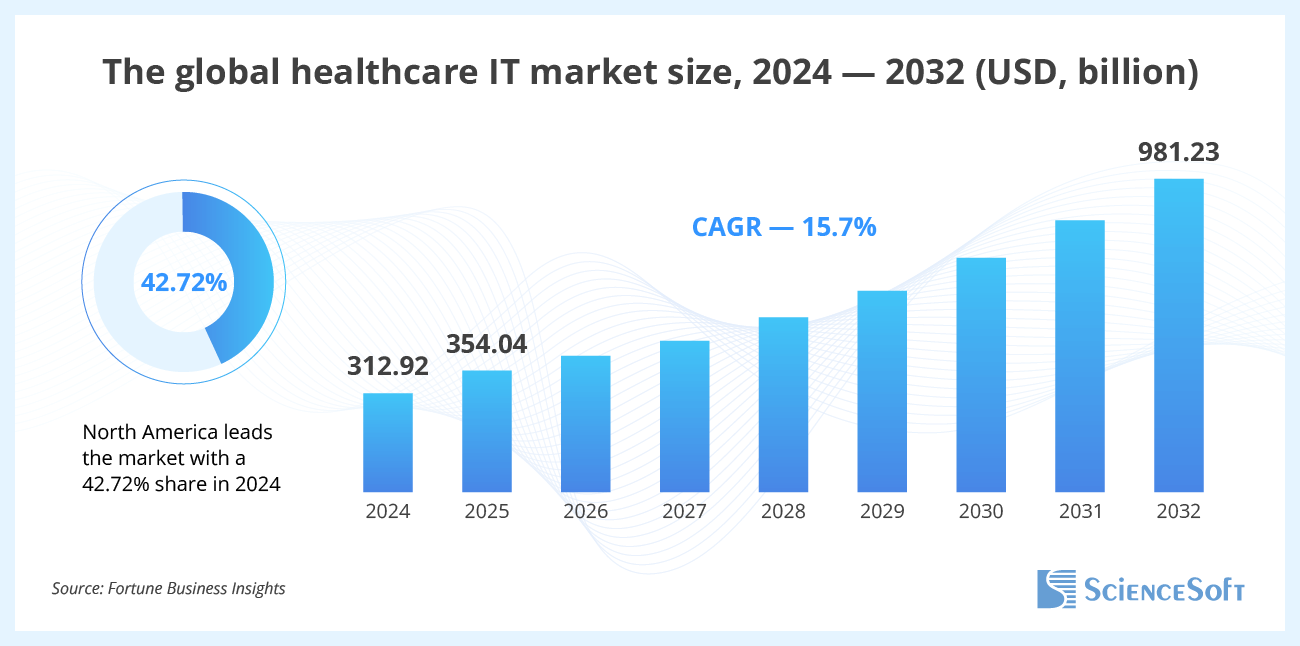
The global healthcare IT market size was valued at $312.92 billion in 2024. It is projected to grow from $354.04 billion in 2025 to $981.23 billion by 2032, at a CAGR of 15.7% during the forecast period. North America leads the market with a 42.72% share in 2024.
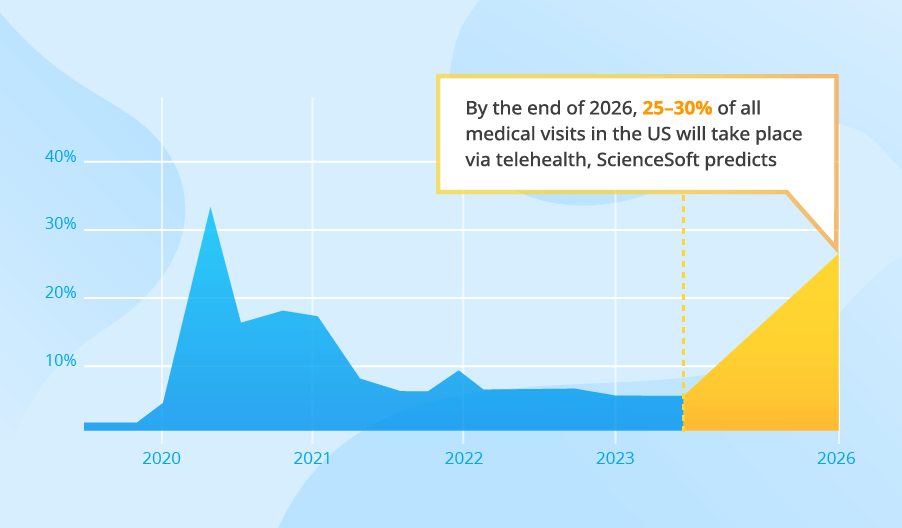
Popular software types include Electronic Health Records (EHR), telehealth, revenue-cycle management (RCM), and analytics. Fortune Business Insights projects that the global EHR market will grow from $31.00 billion in 2024 to $46.63 billion by 2032, while telemedicine is projected to triple, reaching $334.8 billion by 2032. The market for revenue-cycle management software is similarly large. It is valued at approximately $148.8 billion in 2024.
The main driving factors in healthcare software development include aging populations with increasing rates of chronic diseases, medical staff shortages, patient demand for convenience, and payment reforms.
Opportunities for startups
Building EHR or RCM systems from scratch is rarely feasible for startups, as established vendors like Epic and Cerner already dominate this market segment. Startups willing to serve this niche may consider partnering with providers of these solutions and offering specific modules to augment their capabilities, for example, specialized patient engagement, analytics, or automation tools.
The opportunities in areas like telehealth, remote patient monitoring, and disease-specific mobile apps (e.g., for diabetes or heart failure) are growing with the growing number of chronic diseases.
Healthcare Digital Solutions ScienceSoft Develops
Below, you'll find some examples of healthcare software products that our clients request most frequently. We're also ready to support the development of specialized or niche healthcare software of varied complexity to bring your unique vision to life.
Remote patient monitoring software
Telemedicine apps
Medical device software and SaMD
Medical imaging software
Chronic disease management software
Healthcare analytics software
Home health software
EHR software
Medication management software
Revenue cycle management (RCM)
Practice management software
In vitro diagnostics software
Hospital asset tracking systems
Healthcare CRM
Hospital inventory management systems
Mental health apps
Fitness and wellness apps
Diet and nutrition apps
High-Demand Technologies ScienceSoft Uses for Healthcare Software
Medical AI chatbots
AI for medical diagnosis
AI for treatment personalization
AI for patient records
AI for mental health
AI in long-term care
RPA in Healthcare
Medical image analysis
Speech recognition for healthcare
Internet of Medical Things (IoMT)
RFID technology for hospitals
Virtual Reality (VR)
Blockchain for personal health records
Wearable medical apps
AI-powered medical devices
Cloud computing technology
Key Steps to Develop Healthcare Software Products
Our healthcare consultants outlined the key steps involved in developing our healthcare software products. Some details in a project roadmap may differ depending on specific project requirements, but overall, the key stages remain roughly the same.
1.
Discovery
This step lays the clinical, technical, and regulatory foundation for your future healthcare software product. At this stage, it's important to:
- Define the product vision by answering the following questions: what problem the software solves, how clinicians, administrative staff, or patients interact with the software, and what task it is designed for.
- Determine the product's regulatory classification and pathway. Does your solution qualify as SaMD? What regulatory requirements apply? What class of device is it and does it require 510 (k) submission or PMA (premarket approval)? Misjudging your software's medical classification at this step can mean losing months and tens of thousands in rework.
- Map your solution against real-world care workflows. ScienceSoft's consultants analyze how your product will fit into existing systems (e.g., EHR, RIS, LIMS), user roles (e.g., nurses, lab techs, radiologists), and physical environments (e.g., bedside, home, operating room).
- Gather user input to frame usability risks. Business analysts study and interview real users, such as clinicians, administrative staff, or patients, to understand their workflows, needs, and limitations. They define how users will actually use the software and what could go wrong from a usability or safety perspective. This step informs a high-level functional scope and risk assumptions.
- Draft a lightweight functional and regulatory roadmap focusing on what's essential for early success. Instead of exhaustive specifications, the goal here is a prioritized core feature list aligned with your minimum viable compliance obligations, business outcomes, and funding stage.
- Create a decision-grade business case that will show what the product will cost, how it will create value, and why it is worth investing in it based on real-world examples (e.g., what a remote patient monitoring app typically costs and earns), regulatory requirements, and impact on healthcare delivery (e.g., hospital readmission reduction, nurse time saved).
- Identify critical risks early, including technical design issues (e.g., PHI processing, international data transfer), clinical reliability, and validation strategy. This avoids rework and audit issues later.
If your company plans to develop SaMD, it is essential to incorporate a robust quality management system (QMS) into the development process. The selected QMS should support compliance with FDA 21 CFR Part 820 and ISO 13485:2016. If your software is intended for the EU and is expected to interact with pharmaceutical production or related environments, EudraLex Annex 11 may apply under GMP rules.
2.
Technical design
Once your product's clinical goals and compliance scope are defined, solution architects translate them into a lean, resilient system architecture. The main goal is to ensure that the architecture meets regulatory and data privacy requirements (e.g., HIPAA, GDPR, MDR), scales safely as you onboard users, and supports interoperability with third-party systems (e.g., EHRs, RIS, pharmacy platforms).
Architects begin by identifying critical technical priorities, such as real-time performance (e.g., for vitals tracking or alarms), auditability (e.g., traceability of clinical decisions), and integration protocols (e.g., HL7 or FHIR for hospital systems).
Then, they select an architecture style, such as microservices or modular. For example, SaMD solutions with frequent algorithm updates might benefit from containerized microservices with separated risk-class modules.
Next, the solution is broken down into functional modules (e.g., patient onboarding, clinical data ingestion, treatment logic). Each module is mapped to a cloud infrastructure (e.g., AWS or Azure). Dataflows are defined, including inbound (e.g., from wearable devices), outbound (e.g., report exports to EHR), and internal (between modules).
Architects also define the tech stack and data exchange formats, taking into account licensing and maintenance risks (especially for open-source libraries), API standardization and support for medical vocabularies (e.g., SNOMED CT, LOINC), expected validation and qualification requirements for regulatory submissions.
3.
UI and UX design
In healthcare, the user interface isn't just about convenience. It directly affects patient safety and clinical efficiency. At this stage, UI and UX designers focus on reducing cognitive load, minimizing input errors, and guiding users through critical workflows. The design approach depends on who will use the software - clinicians, patients, or both.
For clinician-facing interfaces (e.g., decision support, telehealth control panels, diagnostic input screens), UI and UX teams make the solution:
- Easy to use under pressure via implementing large buttons, logical tab order, and simple navigation.
- Safe by design through pre-filled fields, default options, and validation rules that align with clinical logic.
- Support multitasking with keyboard shortcuts, undo or redo features, and also by making critical information (e.g., vital signs) always visible.
For patient-facing solutions, designers address:
- Digital literacy gaps by using clear language, simple visuals, and step-by-step onboarding.
- Accessibility through compliance with Web Content Accessibility Guidelines (WCAG) 2.2. and ISO 9241 principles, screen reader and keyboard navigation support. For solutions intended for use in the US, the requirements of the Americans with Disabilities Act (ADA) are also considered.
- Emotional usability via stress-aware interactions, error messages that offer clear next steps.
The overall UI and UX design process includes:
- Creating wireframes or clickable prototypes based on the MVP feature set.
- Running usability tests with real users or using feedback from domain experts or compliance consultants when real users are unavailable.
- Following IEC 62366 usability standards, when the solution is likely to qualify as medical device software or SaMD.
4.
Development and testing
For healthcare software, development must do more than deliver features; it must ensure trust, traceability, and regulatory compliance. We recommend the Agile approach with short sprints, but with a critical overlay: development should always be guided by compliance, patient safety, and clinical logic.
At this stage, startups should:
- Break the product into clinically meaningful increments, not just "login" or "chat module," but "vitals capture flow" or "treatment plan adjustment logic."
- Validate logic early with medical experts. For example, in a remote patient monitoring app, ensure alerts and thresholds reflect clinical norms to avoid false negatives or alarm fatigue.
- Include compliance checks in each sprint, e.g., log data flows for HIPAA impact review or document logic behind alerts or treatment decisions for SaMD traceability.
Functional and performance testing ensures software works well on different devices and under different usage loads, which is especially critical for real-time or cloud-connected tools.
Usability testing helps prevent workflow or decision errors, which is particularly important if clinicians will use the software under pressure.
Security testing should be started from sprint one, especially for apps processing PHI or interfacing with hospital systems. Test for issues like weak access controls or insecure code.
If your software connects to a physical device (e.g., a wearable, or alert system), simulate real-world use scenarios. For example, test connectivity drop-offs in home care environments or battery faults in eldercare devices.
5.
Pre-launch activities
Pre-launch activities depend heavily on your product's regulatory classification and deployment environment.
If your software qualifies as a medical device, pre-launch activities may include weeks or even months. Here's what we recommend:
Finalize your technical documentation, including:
- Software architecture diagrams (how your app is built).
- Data flow diagrams (how the data moves through your app).
- Validation reports (proof that your app operates safely and correctly).
- Usability test results (showing it's safe to use according to IEC 62366).
Initiate your premarket submission:
- FDA 510(k) process for the US.
- MDR/IVDR Notified Body review for the EU.
You cannot launch your product widely until you get regulatory approval. Only a very limited pilot use is allowed under a formal clinical investigation protocol or usability testing not involving diagnostic or treatment use.
If your software does not qualify as a medical device (e.g., appointment scheduling), the process is faster, but still requires structured preparation to protect users and data. Here's what you should do:
- Launch a limited-access pilot to internal staff, test clinics, or early adopters.
- Use pilot feedback to refine task flows, onboarding, and language clarity.
- Prepare basic documentation, such as user manuals for clinicians or patients, and support content (chat, FAQs, in-app tutorials).
If your healthcare product qualifies as medical device software or SaMD, you should initiate premarket submission according to the FDA 510(k) program (US) or the requirements of a Notified Body designated under MDR/IVDR (EU). In case of approval, the product can be marketed. If a regulatory authority requests adjustments to the product following a review or pilot rollout, such adjustments should be implemented, and the software resubmitted. Besides, if your app introduces new treatments, clinical trials should be conducted first to prove their efficiency.
6.
Release, support, maintenance, and evolution
After the initial release, focus on continuous product evolution. Gather feedback from real users, such as clinicians, administrative staff, or patients, and adjust the system accordingly to enhance care workflows, patient safety, and engagement. If your solution qualifies as medical device software, monitor for post-market safety signals, manage documentation for audits, and implement updates in line with regulatory changes (e.g., FDA guidance updates or MDR amendments). Track your system’s performance, uptime, and data integrity, especially for apps handling PHI. Enhance your app with strategic new features that will increase its immediate value, such as FHIR-based integrations with EHR systems, predictive analytics modules, AI-powered virtual assistants, in-app communication capabilities, multi-language support, and more.
Why Choose ScienceSoft as Your Tech Partner
- In healthcare IT since 2005.
- ISO 13485, ISO 9001, and ISO 27001 certifications.
- Experience in achieving compliance with the requirements of HIPAA, GDPR, the 21st Century Cures Act, FDA 21 CFR Part 820, FDA 510(k), MDR, IVDR, CEHRT, and 42 CFR Part 2.
- MVP ready for users in 2–6 months.
- Proficiency in high-demand techs: AI, big data, blockchain, IoT, VR, and AR.
- Deep knowledge of data exchange standards such as FHIR, XDS/XDS-I, NCPDP SCRIPT, CCDA, and USCDI.
- Expertise in clinical coding and terminology standards (ICD-10, CPT, SNOMED CT, LOINC, and RxNorm).
Our awards and partnerships
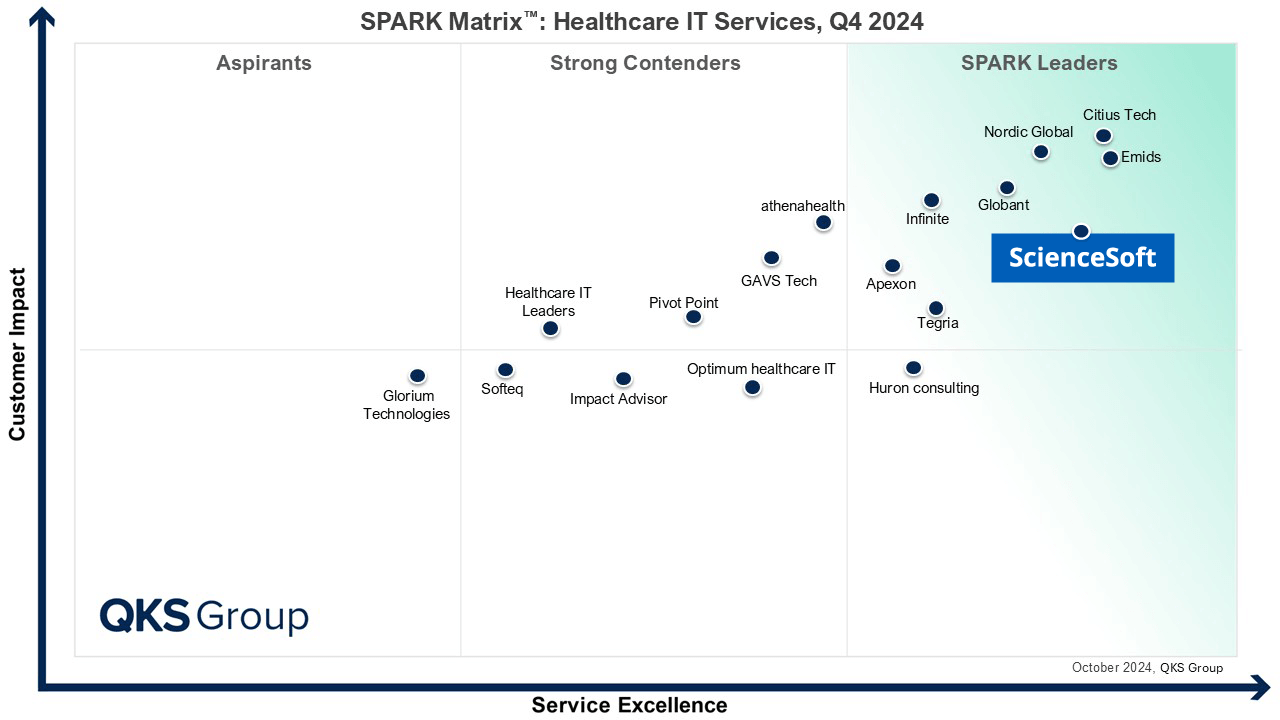
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row
Recognized on Newsweek’s 2025 America’s Most Reliable Companies List
Recognized by Health Tech Newspaper awards for the third time

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Named Best in Class in Medical Device Connectivity by Frost & Sullivan (2023)
Listed in IAOP’s 2025 Global Outsourcing 100 for the 4th year running
ISO 13485-certified quality management system
ISO 27001-certified security management system
Sourcing Models for Healthcare Product Development
Technologies ScienceSoft Uses to Develop Healthcare Products
ScienceSoft's experts typically apply the following technologies to deliver reliable healthcare software and keep IT costs under control.
Low-code development
Clouds
DevOps
Containerization
Automation
CI/CD tools
Monitoring
Costs of Healthcare Software Product Development
When considering the development of a healthcare software product, apart from the universal cost drivers like product complexity and scope, expected number of users, and APIs, take into account the following cost drivers:
- Regulatory classification. It determines the depth of validation, risk analysis, and documentation. For example, a clinical decision support system classified as SaMD must follow strict data protection and audit trail requirements, which require additional development efforts compared to a general wellness tool that needs minimal regulatory oversight.
- Real-time clinical logic. Building and validating decision-support or alert logic demands rigorous testing and traceability.
- Data interoperability. Supporting HL7 or FHIR, or syncing with EHR systems like Epic or Cerner, adds substantial effort to design, test, and certification.
- Security and privacy readiness. HIPAA and GDPR-compliant data encryption, access controls, and audit logging add both technical and documentation work.
- Usability under regulation. If usability affects clinical safety (e.g., dosing UI), compliance with IEC 62366 may be required. This means more effort in UI/UX design and testing.
- Patient-facing features. These often need accessibility support (WCAG 2.2), digital literacy design, and multilingual content. For example, a mobile health app for older adults or those with disabilities must include multiple accessibility features, which adds to the design and development time and costs.
- Testing requirements. While MVPs may get by with simulated data, regulated apps need formal verification, traceable test cases, and usability trials.
The general rule is that the more your healthcare product deals with real clinical decisions, or patient data, the more time and cost you should expect for both development and validation.
Average MVP Costs
The cost of MVP development ranges widely between $105,000 and $300,000 for a SaaS MVP. Typically, MVP development costs make up 10–50% of the overall full-scale software development.